Elements
In the past both history and exam have been required for code selection. With the new changes both history and exam will need to be documented but will not affect the code selection. There will be no minimum requirement, beyond establishing medical necessity and the standard of care. Providers will be able to select the E/M service level either by the level of medical decision making or by the total time spent performing the service on the day of the encounter.
The history and/or examination portion of the 2021 E/M guidelines explains that office and other outpatient E/M services include a medically appropriate history and/or physical examination, when performed. “Medically appropriate” means that the physician or other qualified healthcare professional determines the nature and extent of any history or exam for the service performed. The history and exam guidelines also specify that the “care team” may collect information and the patient or caregiver may provide information, such as by portal or questionnaire. The reporting provider must review the information given.
MDM
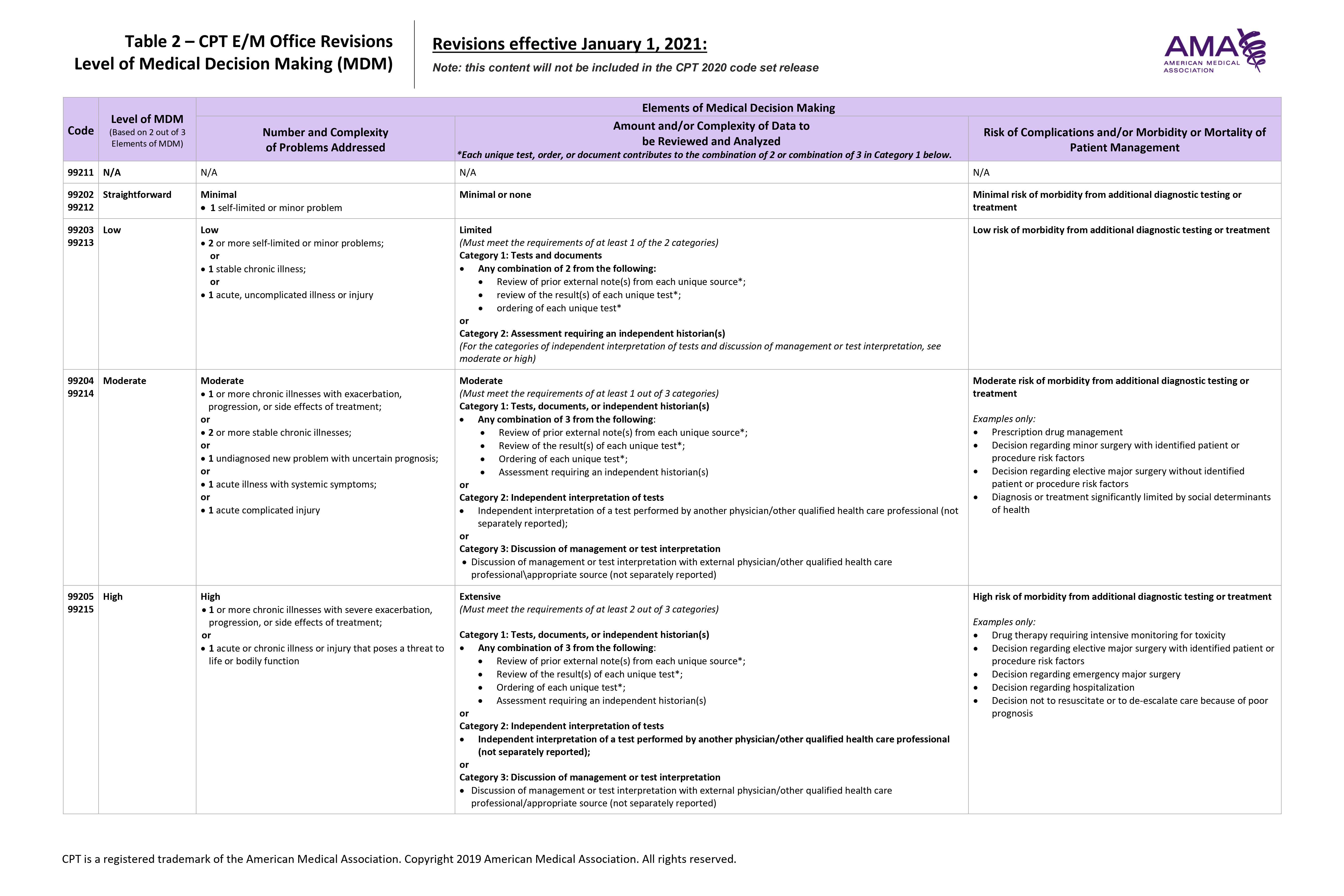
In the 2021 CPT manual there will be a refurbished Table of Risk, which will be known as “The Level of Medical Decision Making Table,” and will assist in calculating the complexity of medical decision making for accurate code selection. The updated table will assist providers and coders in scoring the level of service based on the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
The new table will have slightly different named columns. The columns will be:
- Level of MDM
- Number and Complexity of Problems Addressed
- Amount and/or Complexity of Data to be Reviewed and Analyzed
- Risk of Complications and/or Morbidity or Mortality of Patient Management
Number and Complexity of Problems Addressed – based on both the number and severity of the problems. This will combine the points in Box A and the “presenting problem” column in the current table of risk. This differs from the 2020 guidelines, where the guidelines instead refer to “the number of possible diagnoses and/or the number of management options.” The 2021 guidelines also expand on a 2020 rule by clarifying that comorbidities and underlying diseases should not be considered when selecting the E/M level “unless they are addressed and their presence increases the amount and/or complexity of data to be reviewed and analyzed or the risk of complications and/or morbidity of patient management.”
Amount and/or Complexity of Data to be Reviewed and Analyzed – will have the most notable change and will have the largest impact on code selection. The 2021 guidelines expand this section significantly:
- Category 1: the amount and/or complexity of data to be reviewed and analyzed is based on each “unique” test, order or document that contributes to a combination of two or three points in the new grid.
- Category 2: requires an independent interpretation of tests performed by another physician or provider. This means that if a physician reviews outside tests (MRI, etc.) they can be documented. However, if a test, such as an EKG is performed in the office (which is billable and includes interpretation) this cannot be counted.
- Category 3: includes “discussion of management or test interpretation”. The discussion must occur with an external physician or other provider and is not separately reportable.
Risk of Complications and/or Morbidity or Mortality of Patient Management – will include a more general description for the straightforward and low levels of risk, whereas the moderate and high risks will be supplemented with examples pertaining to the level of risk for each to better assist with code selection.
2021 MDM Terms and Definitions
Problem addressed – the provider must evaluate or treat the problem. Consideration of further testing that is decided against because of risks involved or patient choice counts as addressed. A note stating that another professional is managing a problem does not count as addressed. There must be additional assessment or care coordination. Another area that does not qualify as addressing the problem is referral without evaluation (using history, exam, or diagnostic studies) or considering treatment.
Self-limited or minor problem – a problem that runs a definite and prescribed course, is transient in nature, and is not likely to permanently alter health status. This term is relevant for straightforward MDM codes 99202 and 99212. Note that “or has a good prognosis with management/compliance” has been deleted from the 2021 guideline text regarding this definition.
Risk – related to probability of something happening. Risk and probability are not the same for E/M coding purposes. High probability of a minor adverse effect may be low risk, depending on the case. The terms high, medium, low, and minimal risk are meant to reflect the common meanings used by clinicians. For MDM, base risk on the consequences of the addressed problems when they’re appropriately treated. Risk also comes into play for MDM when deciding whether to begin further testing, treatment, or hospitalization.
External physician or other qualified healthcare professional – someone who is not in the same group practice or is classified as a different specialty or subspecialty. Review of external notes is included in the office/outpatient E/M codes for levels 3 to 5. Discussion with an external provider is included in levels 4 and 5.
Independent historian – family member, witness, or other individual who provides patient history when the patient cannot provide a complete history, or the provider thinks a confirmatory history is needed. Assessment requiring an independent historian is included in office/outpatient E/M levels 3 to 5.
Social determinants of health (SDOH) – economic and social conditions that influence health. The 2021 MDM table references SDOH as an example of moderate risk from additional diagnostic testing or treatment because SDOH, like housing insecurity, may limit those options.
Drug therapy requiring intensive monitoring for toxicity – Per the 2021 CPT MDM table this is an example of high risk of morbidity from additional diagnostic testing or treatment. To be sure the case being coded qualifies as intensive monitoring for toxicity, review the following conditions listed in the guidelines:
- The drug can cause serious morbidity or death
- Monitoring assesses adverse effects not therapeutic efficacy
- The type of monitoring used should be the generally accepted kind for that agent, although patient-specific monitoring may be appropriate, too
- Long-term or short-term monitoring is acceptable
- Long-term monitoring occurs at least quarterly
- Lab, imaging, and physiologic tests are possible monitoring methods, where history and exam are not
- Monitoring affects MDM level when the provider considers the monitoring as part of patient management
- An example of drug therapy requiring intensive monitoring for toxicity is testing for cytopenia (reduction in the number of mature blood cells) between antineoplastic agent dose cycles
Morbidity – a state of illness or functional impairment that is expected to be of substantial duration during which function is limited, quality of life is impaired, or there is organ damage that may not be transient despite treatment.


